Cugell Fellows
Meet the individuals currently in the Pulmonary and Critical Care Cugell Fellowship Program.

Claudie Bosc, PhD
PhD: University of Toulouse III, France
Mentor: Navdeep S. Chandel, PhD
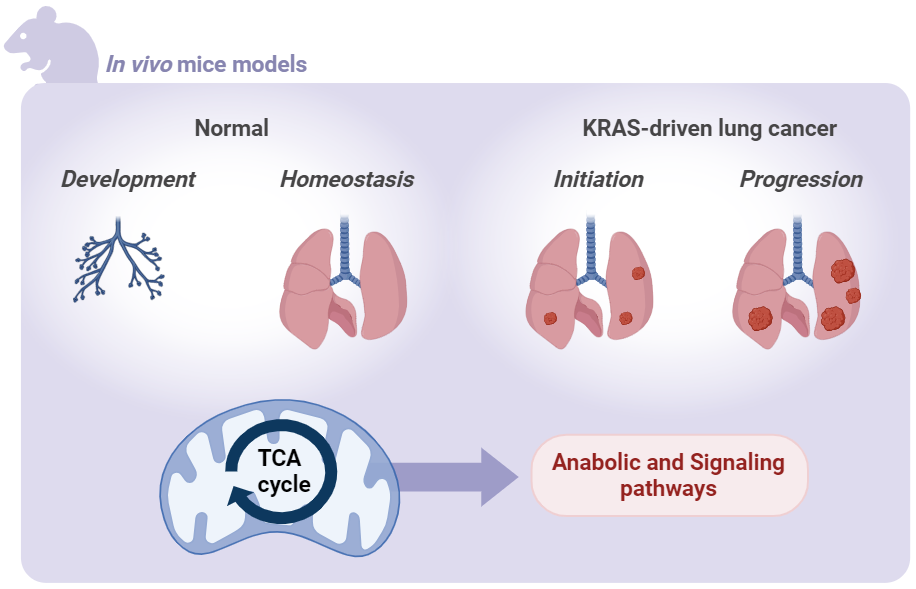
Transformation of normal cells into cancer cells imposes a metabolic demand that is sustained through alterations in critical metabolic pathways and nutrient availability in the tumor microenvironment. Previously, Navdeep S. Chandel, PhD and his group have shown that the electron transport chain (ETC) is necessary for in vivo tumor initiation and progression. However, the exact reasons behind the crucial role of the ETC in tumor growth remain unclear. Claudie Bosc hypothesizes the ETC maintains oxidative tricarboxylic acid (TCA) cycle activity by replenishing NAD+ and converting succinate to fumarate for production of lipids, nucleotides, heme, and glutathione, thus supporting tumor growth. However, a comprehensive understanding of the importance of the TCA cycle in tumor growth has not been achieved thus far. Under Chandel’s mentorship, Bosc will focus her investigation on malate dehydrogenase 2 (MDH2), a key enzyme involved in the final step of the TCA cycle, to explore its significance in tumor growth in a mouse model of lung adenocarcinoma. These investigations will enhance our understanding of the necessity of mitochondrial metabolism dependent TCA cycle in supporting lung tumor growth and normal lung epithelial cell function.

Atsushi Suzuki, MD, PhD
MD: Nagoya City University School of Medicine, Aichi, Japan
PhD: Nagoya University Graduate School of Medicine, Aichi, Japan
Mentor: Alexander V. Misharin, MD, PhD
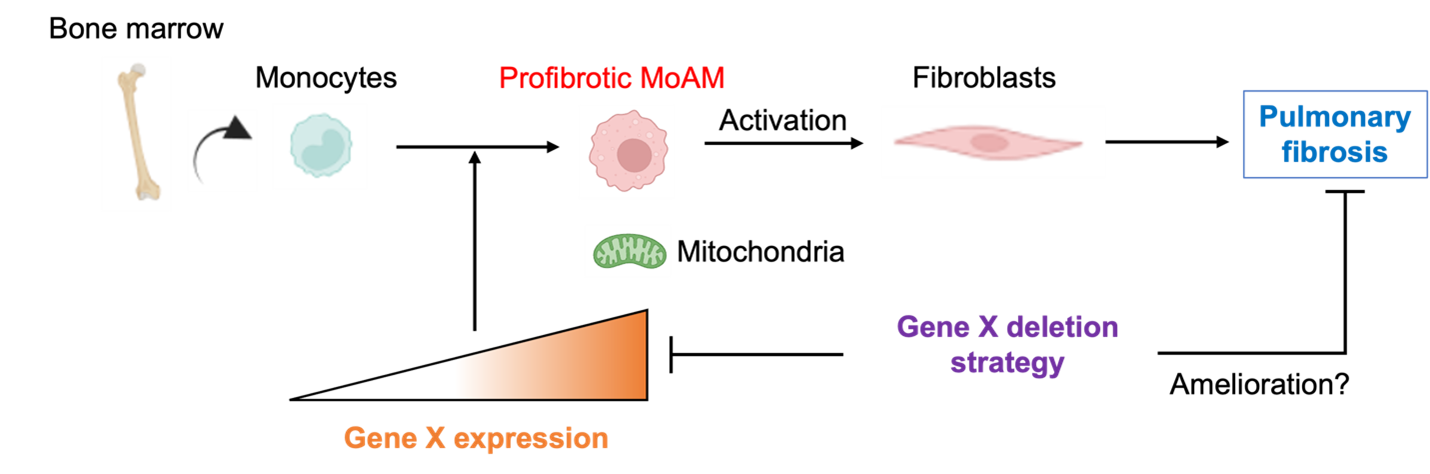
Pulmonary fibrosis is a chronic, progressive lung disease with a poor prognosis, affecting 100,000 individuals in the United States and 3 million people worldwide. Given the limitations of current treatments, Atsushi Suzuki's objective is to discover novel targets for therapy. In this project, Suzuki will focus on monocyte-derived alveolar macrophages (MoAMs), which play a causal role in the development of pulmonary fibrosis. Using modern genomic techniques, he has identified a novel molecule specifically expressed in these cells. Under the mentorship of Alexander V. Misharin, MD, PhD, Suzuki will investigate whether this molecule controls the survival of these pathogenic cells by modulating their mitochondrial metabolism, leading to deterioration in a murine pulmonary fibrosis model. This research has the potential to develop novel, highly specific therapies for patients with pulmonary fibrosis.

Qianli (Iris) Liu
Mentor: Benjamin D. Singer, MD
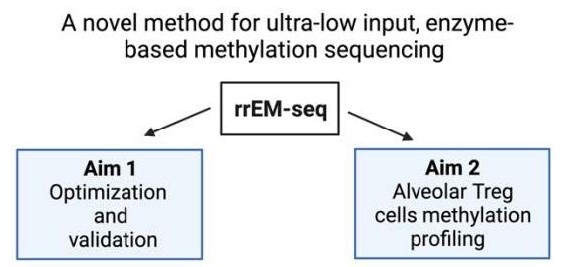
Severe pneumonia represents the leading cause of death due to infection among patients admitted to intensive care units in the United States. CD4+Foxp3+ regulatory T (Treg) cells play an essential role in promoting lung tissue repair following acute injury. In murine models the pro-repair functions of Treg cells depend on maintaining lineage-specific DNA methylation patterns within the Treg cell-specific super-enhancers. Alveolar Tregs cells in patients with severe pneumonia also display the Treg cell-specific methylation signatures predicted by mouse models. Commonly used bisulfite-based procedures for DNA methylation sequencing can degrade DNA, worsening signal-to-noise ratios in samples with low DNA input. Enzymatic methylation sequencing (EM-seq) has been proposed as a less biased alternative for methylation profiling with greater genome coverage. However, whether this technology could be adapted for low-input reduced representation methylation sequencing remains unknown. Iris Liu hypothesizes that reduced representation enzymatic methylation sequencing (RREM-seq) will allow DNA methylation profiling of low-input alveolar Treg cells in bronchoalveolar lavage fluid samples obtained from patients with severe viral pneumonia. In the laboratory of Benjamin D. Singer, MD, Liu will use patient samples and murine CD4+ T cells to test her hypothesis. This project will establish a novel method for high-fidelity reduced representation methylation sequencing with low inputs and determine whether Treg cell-specific methylation signatures can be used to predict clinical outcomes in patients with severe viral pneumonia.

Karen Lou
Mentor: Karen M. Ridge, PhD
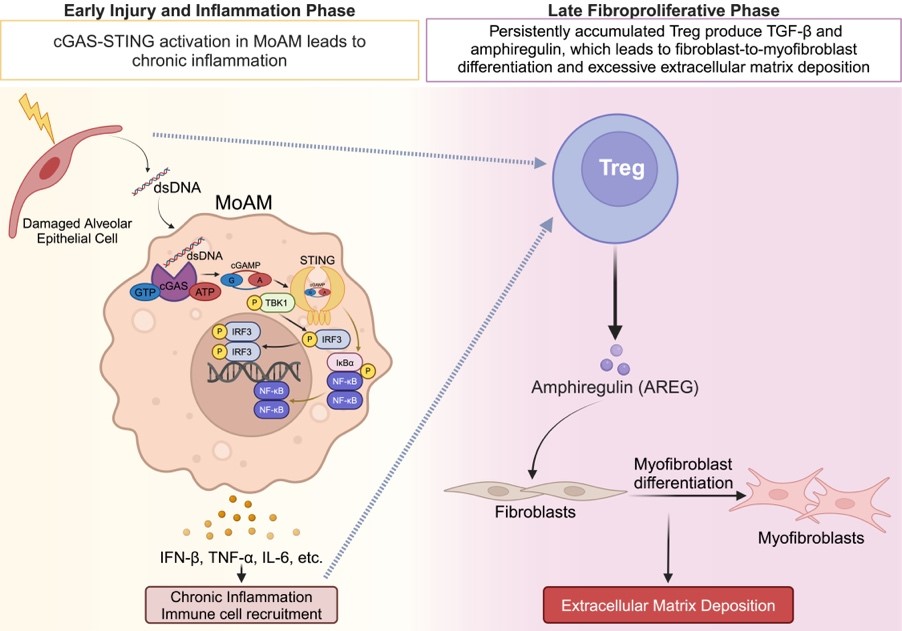
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, lung-restricted disease that leads to increased collagen deposition, disrupting gas exchange and leading to a high mortality rate. At present, there are two FDA-approved drugs for IPF treatment; unfortunately, both drugs only slow the disease progression. The presence of extracellular double-stranded DNA (dsDNA) is a prognostic biomarker of IPF. However, the cell type responsible for dsDNA sensing and subsequent molecular mechanisms contributing to pathogenesis of pulmonary fibrosis are not fully elucidated. Karen Lou hypothesizes that during the injury and inflammation phase, damage to alveolar epithelial cells increases dsDNA, activating the cGAS-STING pathway in monocyte-derived alveolar macrophages (MoAMs) leading to persistent inflammation. Recently, it was reported that patients with progressive IPF have a higher proportion of CD4+Foxp3+ regulatory T (Treg) cells, which is associated with a higher mortality rate, compared to patients with non-progressing IPF. Treg cells produce amphiregulin (AREG), a pro-epithelial repair molecule. Lou hypothesizes that during the late fibroproliferative phase, production of AREG by Treg cells will promote fibroblast-to-myofibroblast differentiation leading to increased collagen deposition and lung fibrosis. In the laboratory of Karen M. Ridge, PhD, Lou will use a a clinically relevant murine model of bleomycin-induced pulmonary fibrosis, characterized by an injury and inflammation phase and a fibroproliferative phase, to test these hypotheses.